Story Highlight
– IXCHIQ vaccine restricted for over 60s and specific conditions.
– 28 serious adverse reactions reported, three fatalities observed.
– Temporary pause on use for people aged 65 and over.
– Risk assessment advised before administering IXCHIQ vaccine.
– Chikungunya transmitted by mosquitoes, causes severe joint pain.
Full Story
The Medicines and Healthcare products Regulatory Agency (MHRA) has implemented notable changes regarding the use of the IXCHIQ vaccine, with specific recommendations aimed at protecting vulnerable groups. Following an extensive safety review, the agency has advised against administering this vaccine to individuals aged 60 and above, as well as to those with particular health issues that could amplify the risks associated with vaccination.
The IXCHIQ vaccine, designed to combat the Chikungunya viral infection, has come under scrutiny after reports emerged indicating 28 cases of severe adverse effects, three of which were fatal. While the vaccine is deemed appropriate for a segment of the population aged between 18 to 59, it is only suitable for those who do not have any of the aforementioned health conditions.
Originally approved by the MHRA in February 2025, IXCHIQ has seen its usage temporarily paused for older individuals since June of the same year, pending a careful evaluation of related safety data. The ongoing review has now culminated in a definitive advisory that highlights the necessity for medical professionals to ensure a comprehensive risk assessment prior to vaccination.
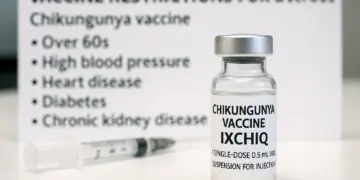
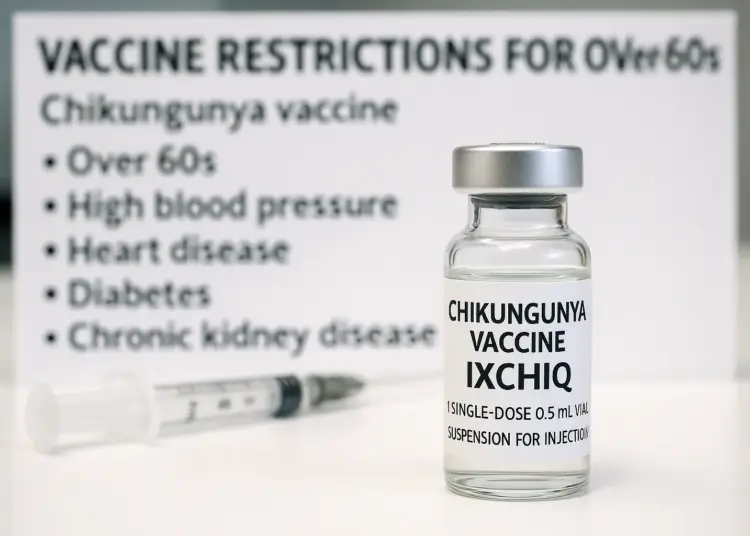
Those advised against receiving the IXCHIQ vaccine include individuals aged over 60, as well as those with the following conditions:
– Hypertension
– Heart disease
– Diabetes
– Chronic kidney disease
Further precaution is recommended for people who are immunodeficient, those who have undergone immunosuppression, and anyone with a history of disorders related to the thymus gland or who have had a thymectomy.
These updated guidelines stem from recommendations provided by an independent expert advisory committee established by the government, reflecting a commitment to patient safety in the face of emerging safety information.
Given that there is no specific antiviral treatment available for preventing Chikungunya, travellers to regions where the virus is endemic are typically advised to receive vaccinations that have been authorised for use within the UK. The two vaccines available are IXCHIQ and another vaccine named Vimkunya.
Chikungunya itself is a viral disease primarily transmitted through mosquito bites and was first recognised during an outbreak in Tanzania in 1952. The term “Chikungunya” originates from the Makonde language, translating to “that which bends up”, a reference to the severe joint pain characterising the illness. While some individuals may not display symptoms, others can suffer from significant consequences due to the virus.
Typically, the initial phase of the infection is marked by the onset of a high fever and debilitating pain in multiple joints, which may be accompanied by swelling or skin rashes. While symptoms generally resolve within approximately ten days, most patients are expected to make a full recovery, as outlined in guidance from Gov.uk.
It is worth noting that Chikungunya is not spread from person to person through conventional interactions such as coughing or sneezing. Instead, the virus is present in subtropical and tropical regions worldwide, including areas across the Americas, Africa, Southeast Asia, India, and the Pacific.
In the wake of these developments, healthcare providers are being reminded to exercise heightened vigilance when considering the IXCHIQ vaccine for patients with pre-existing health concerns. The MHRA’s recommendations serve as a crucial reminder of the necessity for ongoing monitoring and evaluation of vaccine safety, especially as new data emerges.
As public health officials continue to address the risks associated with Chikungunya, the focus remains on ensuring that appropriate measures are taken to protect those most vulnerable. As travel to affected regions becomes increasingly common, understanding the dynamics of Chikungunya transmission and ensuring safe vaccination practices are imperative for safeguarding public health.
Healthcare professionals are being encouraged to remain informed about the latest developments regarding both the IXCHIQ and Vimkunya vaccines, along with their respective safety profiles. As part of their due diligence, clinicians should engage patients in discussions about vaccination options while taking into account individual risk factors and health histories.
The rapid changes in guidance surrounding the IXCHIQ vaccine underscore the importance of a responsive healthcare system prepared to adapt to new information. The objective remains clear: to balance the need for protecting individuals against Chikungunya with the imperative of ensuring patient safety through informed decision-making.
In conclusion, the ongoing review and adjustment of vaccination guidelines reflect a broader commitment to health safety. As research continues and our understanding of vaccine efficacy and safety evolves, the medical community is poised to implement these findings in a manner that prioritises the well-being of patients and communities alike.
Our Thoughts
The incidents surrounding the IXCHIQ vaccine highlight critical areas for improvement in vaccine safety protocols and risk assessment procedures. To prevent adverse reactions, healthcare professionals must adhere to a thorough risk assessment framework as stipulated by the Health and Safety at Work Act 1974. This involves ensuring that proper safety protocols are established, especially when administering vaccines to vulnerable populations, such as individuals over 60 or those with specific health conditions.
Additionally, the Medicines and Healthcare products Regulatory Agency (MHRA) should enhance pharmacovigilance systems to monitor and respond to safety data more proactively. Regulations breached may include the requirement under the Medicines Act 1968 to ensure that medicines are safe and effective before they are authorized for public use.
Key safety lessons include the need for robust pre-approval testing, ongoing safety monitoring, and clear guidance for healthcare professionals regarding contraindications. Future incidents can be mitigated by implementing stricter guidelines for vaccine administration, ensuring that healthcare providers are adequately trained and aware of vulnerable demographics, and that patients are thoroughly informed of potential risks before vaccination.