Story Highlight
– Chikungunya vaccine IXCHIQ faces new usage restrictions.
– Serious adverse reactions reported: 28 cases worldwide.
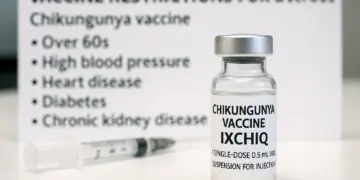
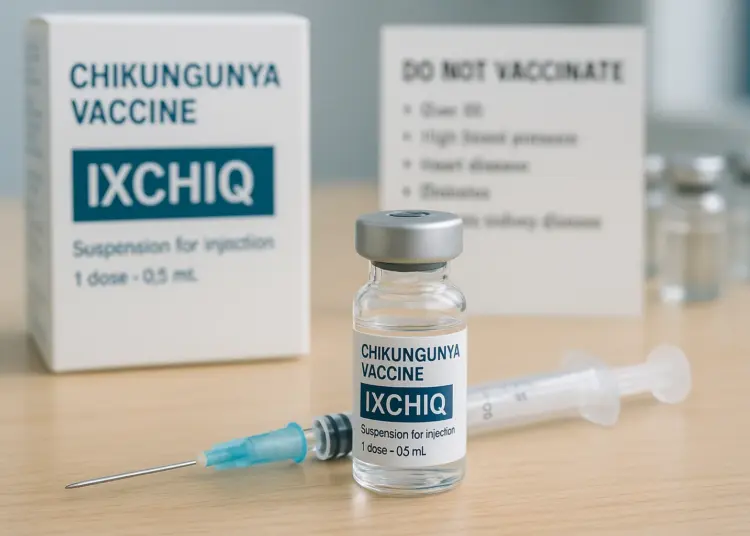
– Over 60s and those with specific conditions advised against vaccine.
– Healthcare professionals must assess risks before vaccination.
– Chikungunya virus transmitted by mosquitoes; symptoms include fever.
Full Story
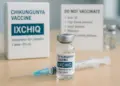
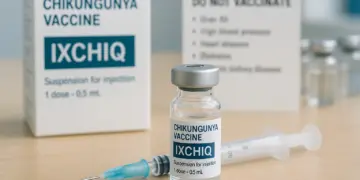
Significant changes have been implemented concerning the IXCHIQ vaccine for Chikungunya, following a review by the Medicines and Healthcare products Regulatory Agency (MHRA). The agency has taken steps to restrict the vaccine’s use, particularly for individuals aged 60 and above, as well as for those with certain pre-existing health conditions.
The announcement comes in light of concerns raised by recent global safety data that identified 28 cases of serious adverse reactions linked to the vaccine, including three fatalities. As a result, recommendations now stipulate that the vaccine should not be administered to older adults or those with specific health issues, such as high blood pressure, heart disease, diabetes, and chronic kidney disease. The vaccine, however, continues to be deemed suitable for individuals aged between 18 and 59 years who do not present any of the aforementioned health challenges.
Originally approved by the MHRA in February 2025, the IXCHIQ vaccine encountered scrutiny as early as June of the same year. During that period, the agency instituted a temporary suspension regarding its use for those aged 65 and older while further evaluations were conducted to ensure safety standards.
Healthcare professionals are now being urged to carry out thorough risk assessments before administering the IXCHIQ vaccine, especially in cases where individuals may have multiple underlying chronic conditions. Additionally, people with compromised immune systems, a history of thymus disorders, or those who have undergone thymectomy are advised against getting vaccinated.
Currently, there are no specific medications available to prevent Chikungunya virus infection. Travellers to regions where the virus is prevalent are typically advised to receive one of the two vaccines approved in the UK. Along with IXCHIQ, another option is the Vimkunya vaccine.
Chikungunya is a viral disease primarily spread through mosquitoes, with its first documented outbreak occurring in Tanzania in 1952. The term ‘Chikungunya’ originates from the Makonde language, translating to “that which bends up,” a reference to the debilitating joint pain experienced by many infected individuals.
While some people infected with the Chikungunya virus may show no symptoms, others can experience severe illness. The virus is notable for being occasionally misdiagnosed as other conditions, such as dengue fever. Symptoms typically begin with a sudden onset of fever and severe joint pain, which can be accompanied by swelling and rashes. According to guidance from Gov.uk, symptoms usually resolve within about ten days, and most patients eventually recover completely.
Importantly, Chikungunya is not transmitted from person to person through everyday interactions, such as coughing, sneezing, or physical contact. The virus is primarily found in subtropical regions across the Americas, Africa, Southeast Asia, India, and the Pacific Islands, posing a risk to populations living or travelling to these areas.
In light of these recent developments, healthcare providers are reminded of their responsibility to stay informed about vaccine-related information. Training and updates on vaccination best practices can significantly contribute to safeguarding public health.
Local health authorities and travel health clinics are preparing to deliver this information to at-risk populations, ensuring that people are fully informed regarding the implications of vaccination and the risk of Chikungunya infection.
As Chikungunya continues to be a public health concern, healthcare professionals and public health officials will likely increase their efforts to educate the community on preventive measures. Encouraging protective actions, such as avoiding mosquito bites through the use of repellent and wearing suitable clothing, is key to minimizing the risk of infection.
As the situation develops, monitoring of the IXCHIQ vaccine’s safety profile will remain crucial. Public health responses will likely adapt based on emerging data and ongoing assessments, which continue to be provided by independent advisory committees.
The adjustments to the vaccination guidelines serve as a reminder of the importance of vigilance in vaccine safety and public health policy. Individuals concerned about the Chikungunya vaccine or their health status are encouraged to consult with their healthcare providers for tailored advice.
Through careful evaluation and implementation of safety measures, authorities aim to enhance the overall health outcomes for the population while continuing to combat infectious diseases like Chikungunya. As more data become available, the public will remain informed about any changes to vaccination practices and health recommendations.
Our Thoughts
The reported serious adverse reactions to the Chikungunya vaccine highlight critical gaps in safety protocols that could have been addressed to prevent these outcomes. Firstly, a more robust pre-approval risk assessment based on demographic factors like age and pre-existing health conditions could have mitigated risks. The Medicines and Healthcare products Regulatory Agency (MHRA) should have enforced stricter data collection and monitoring of vaccine effects in high-risk populations prior to granting approval.
Following the initial release, timely communication of emerging safety concerns should have been prioritized to ensure that healthcare professionals adequately informed and assessed patients before vaccination. The advice against administering the vaccine to certain groups reflects the need for better risk communication strategies.
Key regulations potentially breached include the Health and Safety at Work Act 1974, which mandates employers to ensure the health and safety of employees and the public, and the Control of Substances Hazardous to Health (COSHH) Regulations, which require risk assessments for substances that could harm health.
To prevent similar incidents, ongoing surveillance and adaptive guidelines based on emerging clinical data are essential, along with rigorous training for healthcare providers on risk assessment and adverse reaction monitoring.