Story Highlight
– MHRA warns about addiction risks of common medications.
– 1 in 4 Brits reportedly use these prescription drugs.
– Stronger labels to indicate potential for dependency issued.
– Black market availability linked to growing social issues.
– Health officials advise against abrupt discontinuation of use.
Full Story
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) has recently issued a significant alert regarding the potential for addiction associated with three commonly prescribed classes of medications. The announcement specifically targets gabapentinoids, benzodiazepines, and z-drugs, prompting health officials to call for heightened awareness of their risks.
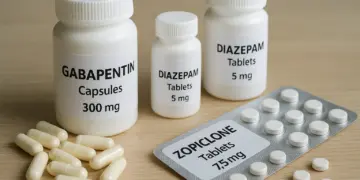
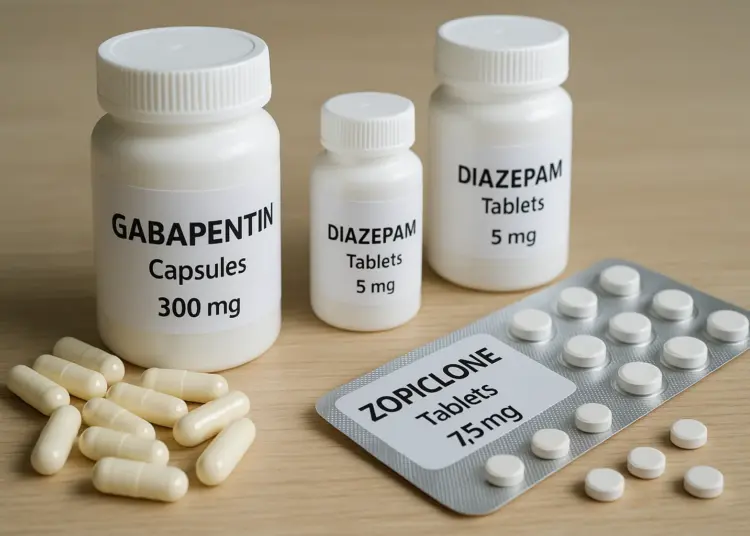
Gabapentinoids, which include medications like pregabalin and gabapentin, are frequently utilised in the management of conditions such as epilepsy and nerve pain. Meanwhile, benzodiazepines, such as diazepam, are often prescribed to tackle anxiety and sleep disorders. Z-drugs, which are primarily indicated for insomnia, encompass commonly known medications like zopiclone, zolpidem, and eszopiclone.
The alert has been instigated by growing concerns regarding the potential for these drugs to induce dependency among users. The MHRA has announced that updated labelling will now clearly stipulate that these medications “may cause addiction, dependence and withdrawal reactions” and will discourage concurrent use with alcohol or opioids.
In a move aimed at safeguarding patients, the revised guidance includes detailed advice on how to responsibly taper off these medications. However, health authorities strongly advise against any abrupt cessation of treatment without prior consultation with a medical professional. Dr Alison Cave, chief safety officer at the MHRA, emphasised the serious risks associated with these widely-used medicines. “Addiction and dependency can happen to anyone taking these medicines, even when used as directed,” she stated. “That’s why we are strengthening warnings, so patients and healthcare professionals can better understand the risks.”
Dr Cave further noted the importance of these drugs in managing certain medical conditions while reinforcing the necessity for patients to have adequate information for safe usage. She advised anyone with concerns regarding their medications or potential side effects to seek guidance from a healthcare professional, underscoring the dangers of discontinuing treatment without adequate medical support—an action that could trigger withdrawal symptoms.
Side effects linked to these medications can vary, with reports highlighting issues such as weight gain and cognitive difficulties. Users may also experience severe withdrawal symptoms that can manifest as nerve pain, heightened anxiety, sleep disturbances, nausea, and excessive sweating. As these medications have gained popularity, their misuse has begun to exhibit notable social consequences. Legal records from various towns indicate a concerning trend, with individuals facing arrests for possession and theft of pregabalin from pharmacies.
The black market has also flourished as a result of increased demand, complicating the landscape further. Patients are reportedly acquiring these drugs through illicit channels, with dangerously contaminated products being sold both online and on the streets. A case in 2024 illustrates the severe risks involved; a man tragically died in Stockton-on-Tees, and seven others were hospitalised after consuming pills from an adulterated supply.
In an alarming testament to the crisis, one individual involved in drug use has been quoted saying, “I am a slave to crack cocaine, Zopiclone and Pregabalins.” He illustrated the lengths some will go to obtain substances: “I get my drugs from the same person in China. I have them posted, and I know it is good stuff. If people pay cheap prices, they are going to get s***. It’s like anything. If you pay more you get better product.”
This heightened awareness about the dangers of these medications comes in the wake of a 2019 review addressing prescriptions in the UK, which raised alarms about a potential ‘over-medication crisis’. The review found that women were significantly more likely to be prescribed these medications than men, with the prevalence rising notably amongst older age groups.
In light of current circumstances, it is crucial that all adverse effects from medications are reported through the MHRA’s Yellow Card Scheme. This initiative, established in the 1960s, provides a platform for healthcare professionals and patients alike to disclose adverse reactions that may arise from both prescription and over-the-counter drugs. Such reports play a pivotal role in the regulatory processes, potentially leading to enhanced warnings, product reviews, or even the removal of harmful substances from market circulation.
For those contemplating the use of pregabalin, it is essential to acknowledge that it is primarily intended for adult patients and may be unsuitable for individuals over the age of 65 or children under 18. The NHS stresses the need for discussions with healthcare providers regarding potential allergic reactions, prior substance misuse, pregnancy or breastfeeding status, dietary restrictions related to sodium, and existing respiratory conditions before commencing treatment.
As the conversation continues around the risks associated with these medications, both healthcare providers and patients must work collaboratively to ensure safety and proper management of these substances, highlighting the importance of informed and cautious usage.
Our Thoughts
The issues surrounding gabapentinoids, benzodiazepines, and z-drugs highlight several key areas where safety improvements could be implemented. Firstly, stricter prescribing guidelines could have mitigated the risk of dependency; adherence to the Health and Safety at Work Act 1974 mandates that employers must ensure the health and safety of employees, which extends to ensuring that prescribed treatments do not adversely affect patient safety.
Additionally, enhanced patient education regarding the risks and side effects of these medications could have prevented misuse. Providing clear information on the potential for addiction and the importance of medical supervision when tapering off could lead to better patient outcomes and comply with the General Medical Council’s guidelines on consent and patient information.
The misuse of these drugs, leading to social issues and fatalities, demonstrates a breach of the Misuse of Drugs Act 1971, particularly regarding their illegal distribution. Increased monitoring and a more robust system to report adverse effects, such as enhanced use of the MHRA’s Yellow Card scheme, would be beneficial in preventing similar incidents.
Overall, incorporating comprehensive risk assessments and regular reviews of drug usage patterns in the UK could enhance safety and reduce instances of addiction and emergency incidents linked to these medications.