Story Highlight
– Coroner raises alarm on unsafe medicine sales practices.
– Paul Pidgeon died from overdose purchased without checks.
– Wholesaler failed to verify customer’s authorization status.
– Death highlights risks of unregulated medicine sources.
– Booker implements stricter qualification processes post-incident.
Full Story
A coroner has raised serious concerns regarding the insufficient safety measures employed by medicine wholesalers, following the overdose death of a man who purchased pain relief medications without any oversight. Paul Pidgeon, a mechanic from Surrey, had established a business relationship with a wholesale distributor, Booker, owned by Tesco.
In February 2022, Mr Pidgeon made a substantial purchase of over-the-counter painkillers, specifically paracetamol and ibuprofen, through his trade account at the Wimbledon branch of Booker. Just weeks later, he returned to buy additional paracetamol, despite his garage business not being involved in supplying medication to the public. Coroner Anna Crawford’s investigation revealed that there were no apparent checks conducted by Booker to validate Mr Pidgeon’s eligibility for purchasing such drugs.
Tragically, Mr Pidgeon was found deceased in March 2022, with empty packaging for both paracetamol and ibuprofen beside him. An inquiry established that his death was a result of paracetamol toxicity, although determining his intentions leading up to the incident proved challenging.
In her Prevention of Future Death report, Anna Crawford expressed alarm at the practices of Booker in Wimbledon which potentially mirror trends across its wider operations. She highlighted the risk that medicinal products could be sold to individuals not qualified to distribute them to the public. Crawford noted that the possibility of substantial quantities of medicines being sold in single transactions heightens the danger that could lead to further fatalities.
The concerns raised are part of a broader narrative around the accessibility of medications from unregulated sources. Health professionals have consistently warned against individuals obtaining medicines from wholesalers or unreliable online platforms. Despite numerous recommendations aimed at curbing this issue, a lack of stringent regulation continues to facilitate the availability of dangerous medications.
The General Pharmaceutical Council (GPhC), responsible for overseeing pharmacy practices in the UK, advises the public on how to identify legitimate online pharmacies. It requires those that legally dispense medications to display the GPhC logo and registration number prominently on their platforms. Patrons can also verify the legitimacy of a pharmacy by referencing its registration number in the GPhC’s official registry.
In the report, Crawford also noted that Mr Pidgeon suffered from two pre-existing medical conditions—coronary artery disease and hepatic steatosis—which increased his vulnerability to the toxic effects of paracetamol. Hepatic steatosis, commonly known as fatty liver disease, is characterised by an accumulation of fat in the liver, often with minimal symptoms, but exacerbates toxicity by raising blood pressure and enhancing the production of harmful metabolites.
Crawford emphasised the crucial need for wholesale suppliers to ensure that they only supply medicinal products to those who possess the appropriate wholesale distribution authorisation or are otherwise authorised to dispense such products to the public. She urged wholesalers to be diligent in checking the credentials of their customers to prevent potential tragedy in the future.
A copy of Crawford’s findings was shared with Mr Pidgeon’s family, the Medicines and Healthcare Products Regulatory Agency (MHRA), and the chief coroner, all of whom have the authority to implement measures to avert similar incidents.
In response to the coroner’s report, Booker extended its “sincere condolences” to Mr Pidgeon’s family and friends. The company stated that it was unaware of the circumstances surrounding his death, expressing regret that it was not invited to present its case during the inquest. They acknowledged Mr Pidgeon’s long-standing account—which he had held for over twenty years—might have involved different qualification protocols at the time of enrollment, complicating matters of compliance.
Booker mentioned that due to the significant duration since Mr Pidgeon’s initial registration, accessing the original documents he submitted to validate his business was no longer feasible. Moreover, the wholesaler hinted at possible miscommunications during the onboarding process but qualified this by deeming it purely speculative.
Furthermore, Booker claimed that its systems have undergone considerable upgrades since 2022, implementing a stringent qualification process that obligates customers to verify their entitlement to supply medicinal products to the public. The wholesaler expressed confidence that these measures would prevent sales of medications to anyone not duly authorised.
As the conversation regarding pharmacy safety continues, the tragic loss of Paul Pidgeon serves as a stark reminder of the potential dangers linked to unregulated access to medications.
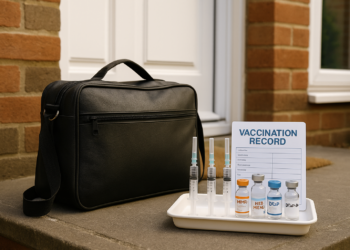





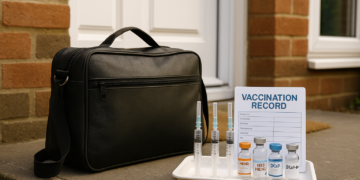








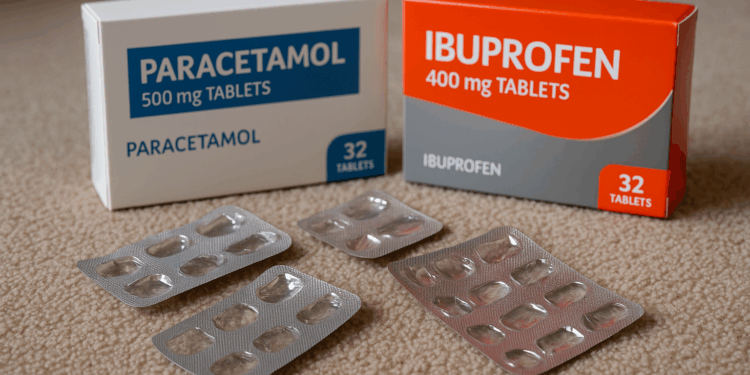




This tragedy highlights the real consequences when controls around medicine supply and verification are insufficient. Suppliers and distributors must ensure robust checks are in place and consistently followed so that medications are only supplied to authorised individuals. Regulators, wholesalers and prescribers need to work together to close gaps that allow dangerous products to reach people without proper oversight. Strengthening verification processes, improving staff training and making clear reporting and audit trails will reduce the risk of similar incidents and better protect public health.
This case highlights a serious gap in the safeguards that should prevent inappropriate access to potentially harmful medicines. Businesses supplying prescription strength products must have robust verification and record keeping to ensure only authorised and trained users obtain them. Wholesalers have a clear duty to implement effective checks and staff training so that policies are followed consistently, and regulators should consider tighter oversight and clearer standards to close loopholes. Public safety depends on a system that balances access to necessary treatments with strong controls to prevent misuse and harm.