Story Highlight
– GLP-1 injections may cause severe acute pancreatitis risk.
– MHRA updates information on weight loss jabs.
– Over 560 pancreatitis cases linked to GLP-1s reported.
– Patients urged to watch for severe stomach pain symptoms.
– Demand for GLP-1s expected to double by 2026.
Full Story
British health authorities have issued a caution regarding the potential for serious side effects associated with GLP-1 weight loss injections, commonly used by numerous individuals across the UK. The Medicines and Healthcare products Regulatory Agency (MHRA) has informed the public that these injections, which include popular brands such as Mounjaro and Wegovy, might carry a small risk of severe acute pancreatitis—a serious inflammation of the pancreas that can result in life-threatening complications.
This advisory follows previous warnings issued by the MHRA last year, which highlighted a troubling link between these weight loss medications and more than 560 reported cases of acute pancreatitis in patients. Among those incidents, at least ten were fatal, prompting the agency to investigate the potential correlation between the medications and the onset of this condition, though causation has not been definitively established.
In response to ongoing safety concerns, the MHRA has revised the product information associated with these GLP-1 medications. The updated guidance aims to enhance awareness among healthcare professionals and patients alike regarding the signs and symptoms of pancreatitis.
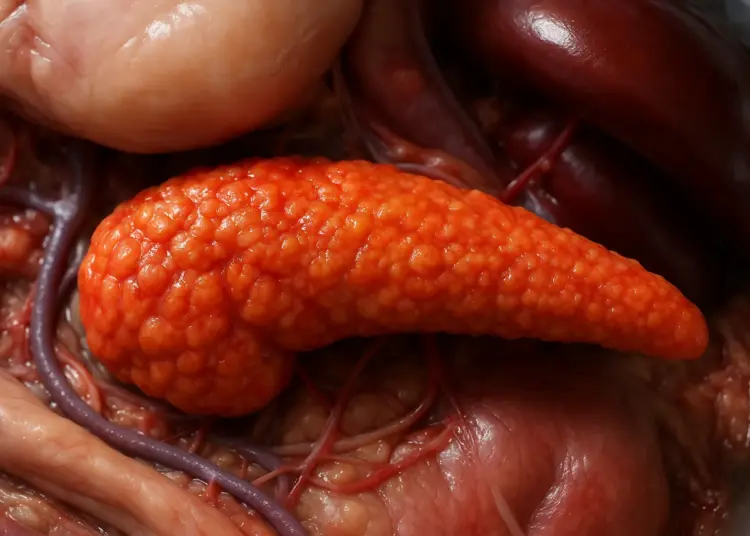
Acute pancreatitis is characterized by a sudden inflammation of the pancreas, a crucial organ situated behind the stomach that plays a vital role in digestion. Symptoms generally manifest as severe abdominal pain, which can extend to the back, alongside nausea and vomiting. While many individuals recover from acute pancreatitis within a week, some may experience a more severe form of the condition that can lead to serious health complications.
The MHRA stated, “Acute pancreatitis is a known, but infrequent side effect of taking GLP-1s. In some extremely rare cases, the complications of acute pancreatitis can be particularly severe.” Severe acute pancreatitis can lead to conditions such as pancreatic necrosis, which involves a loss of blood supply to the pancreas, contributing to tissue death and potential organ failure.
Dr. Alison Cave, the MHRA’s Chief Safety Officer, has emphasized the agency’s commitment to patient safety, noting their continuous monitoring of the safety and efficacy of all approved medicinal products. “For the vast majority of patients who are prescribed GLP-1s, they are safe and effective medicines that deliver significant health benefits,” she stated. Dr. Cave acknowledged the small risk of serious side effects and highlighted the importance of patients and healthcare providers being vigilant for symptoms of pancreatitis.
Patients are advised to be mindful of specific signs indicating potential pancreatitis, particularly severe and persistent abdominal pain, potentially coupled with nausea and vomiting. Those experiencing such symptoms, or who care for someone on GLP-1 therapies, are urged to consult healthcare professionals promptly and report any adverse reactions through the MHRA’s Yellow Card scheme.
The Yellow Card scheme, established in the 1960s, allows for real-time reporting of suspected adverse reactions to both prescription and over-the-counter medications, as well as alternative treatments. Such reports can lead to new warnings being placed on product labels or further action if necessary, such as the withdrawal of a product from the market.
Recent studies from University College London indicate that an estimated 1.6 million adults in England, Wales, and Scotland are using GLP-1 medications to aid in weight management. Projections suggest a doubling in demand for these weight loss jabs is expected by 2026. Despite their generally safe profile, users of GLP-1 injections should remain aware that adverse effects can occur, with mild symptoms such as nausea, constipation, and diarrhoea being relatively common.
Furthermore, professionals emphasize that it is critical for individuals undergoing treatment with GLP-1s to understand the symptoms of severe pancreatitis and to seek immediate medical care should they experience these symptoms. Between 2007 and October 2025, the MHRA received 1,296 Yellow Card reports indicating cases of pancreatitis associated with these weight loss medications in the UK.
In an additional note of caution, the MHRA warns that counterfeit weight loss drugs, often available through unverified online channels, have also been linked to pancreatitis. Such products may contain harmful ingredients that could result in serious side effects.
As part of ongoing safety evaluations, the Yellow Card Biobank project, a collaboration between the MHRA and Genomics England, is working to investigate whether a person’s genetic makeup might influence their risk of developing pancreatitis when using GLP-1 medications. This research aims to help ascertain which patients could be at higher risk for adverse reactions, ensuring that the most suitable and safest treatments are prescribed.
In summary, while GLP-1 medications present significant benefits in weight loss and management, the MHRA’s recent updates serve as a reminder of the importance of awareness and precaution when it comes to treatment. As ongoing research and safety monitoring continue, both patients and healthcare professionals must remain vigilant to safeguard well-being.
Our Thoughts
The risk of severe acute pancreatitis associated with GLP-1 injections, such as Mounjaro and Wegovy, highlights several safety lessons and regulatory considerations.
Firstly, pre-marketing assessments could have been more rigorous, potentially incorporating larger and more diverse population studies to better gauge long-term effects and rare side effects. This aligns with the requirements of the Medicines Act 1968, which advocates for thorough safety evaluations before drug approval.
Secondly, the updates to product information, as mandated by the MHRA, are critical. However, continuous education for healthcare professionals and patients regarding the symptoms of acute pancreatitis could have been emphasized earlier in the marketing phase. The Health and Safety at Work Act 1974 emphasizes the importance of providing sufficient information to mitigate risks associated with health treatments.
Thirdly, there is a need to enhance monitoring post-licensing through systems like the Yellow Card scheme, ensuring prompt reporting and response to adverse reactions. Moreover, mitigating the risks of counterfeit medications, which could contribute to the incidents of pancreatitis, is essential. Strict guidelines on the sale and distribution of medical products must be enforced to protect patients.
In summary, more robust pre-licensing trials, improved ongoing education, and stringent regulations on counterfeits could help prevent similar incidents in the future.