Story Highlight
– MHRA warns of serious side effects from GLP-1 medications.
– 1.6 million people in the UK used GLP-1s recently.
– Seek urgent help for severe abdominal and back pain.
– Risk of acute pancreatitis linked to GLP-1 use.
– MHRA emphasizes patient safety as top priority.
Full Story
The Medicines and Healthcare products Regulatory Agency (MHRA) has issued an important warning regarding the potential risks associated with GLP-1 medications, commonly used for weight management. This comes amid growing usage of such medications, with approximately 1.6 million individuals in the UK having accessed these treatments in the past year.
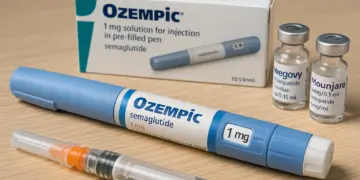
The available medications in this category include Ozempic, Wegovy, and Mounjaro, each of which plays a role in managing weight loss and treating type 2 diabetes. The alert focuses on a notable side effect: acute pancreatitis, a serious condition characterized by inflammation of the pancreas. The MHRA has urged both patients and healthcare providers to remain vigilant for symptoms associated with this condition, particularly severe and persistent abdominal pain that may radiate to the back.
Alison Cave, Chief Safety Officer at the MHRA, emphasized the agency’s continuous commitment to safeguarding patient health. She stated, “Patient safety is the MHRA’s top priority and we continually monitor the safety and efficacy of all licensed medicines.” Her remarks highlight the organization’s proactive approach to identifying and managing potential risks linked with medications prescribed to millions of patients.
The warning serves as a critical reminder for those administering GLP-1 medications to be aware of severe health repercussions. According to the NHS, while many individuals may not experience ongoing issues—typically improving within a week—there remains a risk of developing complications from acute pancreatitis. Symptoms to watch for include not just significant abdominal pain, but also nausea, vomiting, and fever.
To ensure comprehensive awareness, the MHRA has updated product information for healthcare professionals and patients alike regarding these medications. The agency aims to ensure all individuals taking GLP-1s are educated on the associated risks as part of broader efforts to promote informed healthcare decisions. Patients and healthcare providers have been advised to remain alert for indicators of acute pancreatitis and to seek immediate medical attention should symptoms arise.
The MHRA’s announcement aligns with new findings from research undertaken by University College London, which indicates that between early 2024 and early 2025, a substantial number of adults across England, Wales, and Scotland engaged with GLP-1 medications to aid weight loss. This sharp uptick in usage is reflective of a broader trend in weight management solutions, where the appetite-suppressing qualities of these medications have gained popularity.
While GLP-1 therapies have shown to be beneficial for managing diabetes and in weight reduction, they are not without their inherent risks. The MHRA’s caution comes in light of these facts, reinforcing the notion that while the vast majority of patients can safely benefit from GLP-1 medications, awareness of possible side effects remains crucial.
Healthcare experts concur that it is vital for patients to educate themselves on the symptoms of acute pancreatitis. As a point of reference, common signs indicating this condition, as outlined by the NHS, include sudden and severe abdominal pain, which may be accompanied by feelings of sickness or fever. The recommendation is consistent: individuals who experience such symptoms should contact a healthcare professional promptly. If a personal visit to the GP is unfeasible, patients are advised to reach out to NHS 111 for further guidance.
The range of uses for GLP-1 medications extends beyond weight management; Ozempic, for instance, is also deployed for diabetes treatment and for lowering the risk of cardiovascular events. The agency’s directive is a proactive measure that highlights the importance of monitoring and evaluating the safety profile of medications in light of the increasing prevalence of their usage.
As efforts continue to address obesity, diabetes, and associated health conditions, the MHRA’s vigilance serves as a crucial reminder of the complexities involved in medication management. In parallel to the rise in prescriptions for GLP-1s, the potential complications linked to their use warrant careful consideration and ongoing education for both patients and healthcare practitioners.
In conclusion, the MHRA’s recent communication highlights the delicate balance between effective treatment strategies and patient safety. As the healthcare community navigates these concerns, ongoing dialogue, awareness, and support systems will be essential in fostering a safe environment for individuals relying on these medications for weight management and chronic conditions. Healthcare professionals are encouraged to maintain transparency with their patients regarding the benefits and risks of therapies, ensuring that individuals can make informed choices in collaboration with their healthcare teams.
Our Thoughts
To avoid potential incidents related to the use of GLP-1 medications, the following key safety measures could have been implemented:
1. **Improved Patient Education**: Enhanced information regarding the risks of severe side effects, including symptoms of acute pancreatitis, should be provided to patients at the point of prescription. This aligns with the requirements of the Health and Safety at Work Act 1974, which emphasizes the importance of adequate training and information for employees and the public.
2. **Regular Monitoring and Reporting**: The MHRA should enforce more stringent monitoring of medication side effects. Encouraging patients to report adverse reactions via the Yellow Card scheme is vital but should be coupled with proactive follow-ups by healthcare professionals.
3. **Updated Risk Assessments**: Healthcare providers must conduct regular risk assessments concerning the use of medications, as outlined by the Management of Health and Safety at Work Regulations 1999. This includes considering the long-term effects of widely prescribed treatments like GLP-1s.
4. **Enhanced Communication Protocols**: Establishing clearer communication protocols between healthcare providers and patients regarding when to seek help for symptoms could reduce the risk of complications, adhering to the principles of the Medicines Act 1968 regarding the safe distribution of medicines.
By implementing these safety measures, the risk of adverse effects from GLP-1 medications could be significantly reduced.