Story Highlight
– MHRA warns GLP-1 drug users of serious side effects.
– Symptoms include severe stomach pain and nausea.
– Over 1.6 million people in UK use GLP-1s.
– Most patients experience safe, effective results.
– Seek immediate medical help for severe pain symptoms.
Full Story
The Medicines and Healthcare products Regulatory Agency (MHRA) has issued an important update regarding the use of GLP-1 receptor agonist medications, which are increasingly prescribed for weight management. Recent guidance urges both healthcare providers and patients to remain vigilant for a potential, albeit rare, side effect: acute pancreatitis.
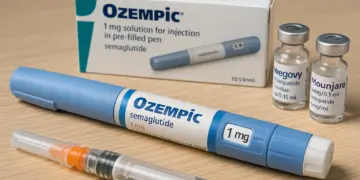
In the UK, approximately 1.6 million individuals have opted for GLP-1 injections, such as Ozempic, Wegovy, and Mounjaro, over the past year as part of their weight loss journey. The MHRA’s announcement serves to enhance awareness around this serious condition that can arise in some patients using these medications. The agency has recommended an immediate medical evaluation for anyone experiencing severe and persistent pain in the stomach or back, which may indicate pancreatitis—an inflammation of the pancreas located behind the stomach.
Patients are advised to seek prompt medical assistance if they notice symptoms such as sudden, debilitating abdominal pain that may radiate to the back, accompanied by nausea and vomiting. While many individuals who develop this condition may recover within a week’s time, the NHS notes that others might face more severe complications.
The MHRA publicised this new guidance on a Friday, updating the product information supplied to healthcare professionals. Alison Cave, the Chief Safety Officer at the MHRA, underscored the organisation’s commitment to patient safety, stating, “Patient safety is the MHRA’s top priority, and we continually monitor the safety and efficacy of all licensed medicines.” She highlighted the overall safety profile of GLP-1 medications, affirming that they bring substantial health benefits for most patients. Cave further added, “Although the risk of developing these severe side effects is very small, it is essential that patients and healthcare professionals remain aware and alert to the symptoms.”
In layperson’s terms, this means that while GLP-1 medications are generally safe, individuals using them should be cognisant of the signs of acute pancreatitis. The agency encourages patients or caregivers to act swiftly if any troubling symptoms manifest, ensuring they are documented through the MHRA’s Yellow Card scheme. This platform allows for reporting adverse reactions or concerns about medication.
For context, Ozempic is primarily indicated for the treatment of type 2 diabetes and cardiovascular health, while both Wegovy and Mounjaro are specifically approved for weight management. According to MHRA statements, GLP-1 medications also play a role in cardiovascular risk reduction for patients with established cardiovascular disease and a body mass index (BMI) of 27 kg/m² or above.
Recent research conducted by University College London revealed an intriguing statistic: between early 2024 and early 2025, an estimated 1.6 million adults from England, Wales, and Scotland had utilised GLP-1s, including semaglutide (found in both Wegovy and Ozempic) and tirzepatide (Mounjaro), primarily for weight loss. This growing usage highlights the increasing tendency of healthcare providers to prescribe these medications as viable options for controlling weight and associated health risks.
However, as is the case with any medication, the benefits are tied to potential risks. The NHS has reiterated that awareness of the signs indicating acute pancreatitis is essential. Common symptoms of this condition include sudden, severe abdominal pain, nausea, vomiting, and a fever exceeding 38 degrees Celsius. The urgency of seeking medical assistance is underscored by the NHS’s advice, recommending that individuals who suddenly develop intense abdominal pain should consult a GP without delay or reach out to NHS 111 for further guidance if immediate consultation is not feasible.
While GLP-1 medications have been heralded for their weight loss properties and potential to alleviate other health concerns, the recently issued warnings serve as a vital reminder for ongoing communication between patients and healthcare providers. The MHRA aims to ensure that patients are well-informed about both the advantages and potential side effects of their treatments, enabling them to make educated choices regarding their health.
As the landscape of medical treatments for obesity and associated health conditions continues to evolve, the importance of patient education and vigilance cannot be overstated. The MHRA’s proactive measures reflect a broader commitment within the healthcare community to prioritise patient safety while harnessing the therapeutic benefits of these innovative medications. Therefore, individuals taking GLP-1 medications should remain informed and engaged with their healthcare teams to achieve optimal outcomes while minimising risk.
Our Thoughts
The recent guidance from the MHRA regarding GLP-1 drugs highlights key areas for improvement in patient safety and communication. To prevent adverse effects like acute pancreatitis, healthcare providers should ensure thorough patient education on the potential side effects of medications. Ensuring that both patients and healthcare professionals are well-informed about the symptoms to monitor could lead to earlier diagnosis and intervention, reducing the risk of severe complications.
Key safety lessons include the importance of vigilant monitoring and timely reporting of adverse reactions through established channels, such as the MHRA’s Yellow Card scheme. Regular training for healthcare professionals on recognising and addressing medication side effects can further enhance patient safety.
Regarding relevant regulations, there may have been a breach of the Medicines Act 1968, which outlines the responsibilities of manufacturers and healthcare providers in ensuring product safety and effective communication to end users.
To prevent similar incidents in the future, a framework for ongoing patient education throughout treatment, coupled with stringent monitoring processes, should be established. Periodic reviews of prescribing practices based on emerging safety data are also vital to ensuring patient safety.